Gastrointestinal nematodes (GIN) affect grazing ruminants causing parasitic gastroenteritis1. Although morbidity or mortality rates are not usually high, GIN infections lead to noticeable economic losses ranging from €11 to €87 million annually, dairy cattle being the most affected sector1,2,3. In addition, resistance to anthelmintics is an increasing problem; thus, more effective methods of control and prevention are needed for keeping these infections below economically-damaging levels4.
The lifecycle of GIN is direct and the climate has an important effect on the species of nematodes present in a given geographic region and in their phenology1. Thus, an increased risk of GIN infection is expected in northern temperate areas due to global warming, causing a rise in clinical disease outbreaks5.
A wide diversity of parasites
Parasitic gastroenteritis is usually caused by a combination of several nematode species with different locations within the host; most of them are host-specific5. Adults of Haemonchus, Ostertagia, Teladorsagia and Trichostrongylus axei are found in the abomasum, whereas those of Bunostomum, Capillaria, Cooperia, Nematodirus and Trichostrongylus are found in the small intestine and those of Chabertia, Oesophagostomum and Trichuris in the large intestine3. The most important species in cattle are Ostertagia ostertagi and Cooperia oncophora1,2 whereas Haemonchus contortus and Teladorsagia circumcincta are the most pathogenic species in small ruminants5,6.
|
|
Abomasum |
Small intestine |
Large intestine |
| Cattle |
Ostertagia, Trichostrongylus axei |
Bunostomum, Capillaria, Cooperia |
Oesophagostomum |
| Small ruminants |
Haemonchus, Teladorsagia |
Nematodirus, Trichostrongylus, Cooperia |
Chabertia, Oesophagostomum, Trichuris |
Gastrointestinal nematodes general lifecycle
The lifecycle of ruminant GIN is direct and follows a similar pattern for most species. Infected animals shed eggs within feces. For most GIN species, the first larval stage (L1) hatches from the egg and develops into an infectious L3 within the feces if environmental conditions are appropriate5. Mild temperatures (5-35ºC) and high fecal water content (60-70%) are needed for larval development, although the optimal development is generally achieved at 18-23 ºC; longer periods of time are needed when temperature is not appropriate, and development can even be suspended7. Afterwards, L3 migrate onto the pasture herbage; some factors can influence the distribution of infective larvae: L3 can be washed down by rainwater or mechanically dispersed by people, machines, animals (including insects) or even fungi3.
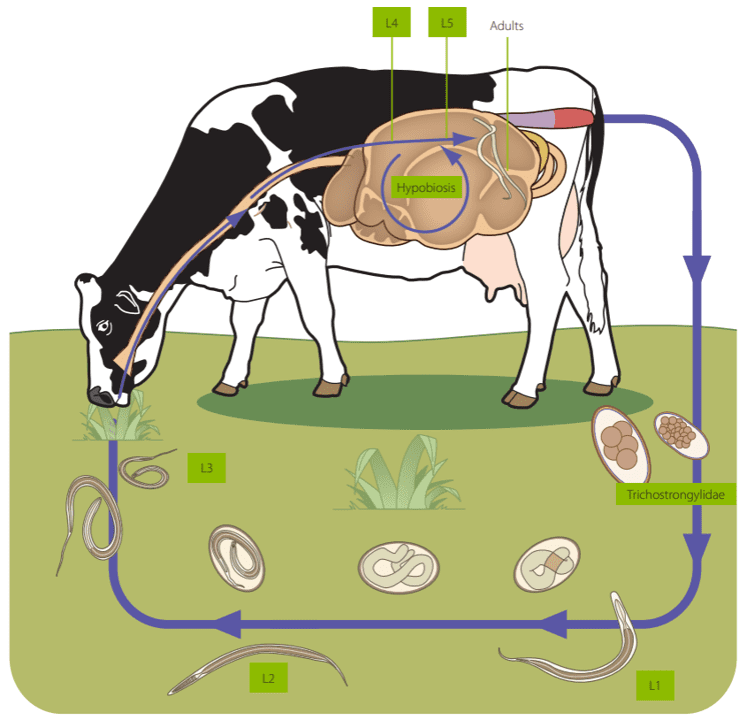
Animals get infected after ingesting L3 when grazing. There is a tissue phase within gastrointestinal mucosa before fifth stage larvae (L5) emerge into the gastrointestinal lumen and become sexually mature3. The prepatent period depends on the species, mainly ranging from 2 to 4 weeks, although it can be as long as 10 weeks3.
GIN strategies for completing their lifecycle
The development of a protective immune response after continuous reinfections leads to the decrease in the number of adults and egg shedding; thus, adult ruminants are carriers of GIN during the winter season and contaminate the pastures with low numbers of eggs during the spring. GIN can adopt different strategies leading to high pasture L3 contamination coinciding with the presence of young naïve ruminants, very susceptible to infection, ensuring transmission from one generation to the next8.
- Overwintering: L3 presents a sheath which protects it from environmental conditions. Thus, some L3 can overwinter in the pasture, even under snow, representing an important infection source for young animals at the beginning of the grazing season. However, the number of overwintering L3 in the pasture decreases during the following warm months (May and June) 3.
- Hypobiosis: in some instances, the development of several gastrointestinal nematodes such as Haemonchus, Ostertagia and Teladorsagia can be arrested (hypobiotic or inhibited phase) in the histotropic L4 stage for periods up to 6 months3. This can be induced when adult GIN burdens are high in the host, allowing the regulation of nematode density in the gastrointestinal tract, or after a host immune response. Nevertheless, it usually occurs under environmental conditions unfavorable to free-living larval stages such as long, severe winters or hot, dry summers; thus, hypobiotic larvae usually resume their development in spring or autumn3,9.
- Periparturient rise: the periparturient reduction of immunity favors the survival of existing GIN in the host, which increases egg production5.
Consequently, young ruminants get infected and shed a large number of eggs a few weeks after infection, having an important role in pasture contamination from June to August. Seasonal dynamics can be influenced by several variables such as climate, altitude rainfall, temperature, stocking density and the age of the animals3.

Global warming consequences
Global warming is expected to lead to a progressive increase in temperatures as well as to extended growing seasons; thus, an increase in the prevalence and intensity of infection by gastrointestinal nematodes, especially haemonchosis, has been predicted in grazing animals in temperate areas of Europe, probably due to the fast development of L3 in summer and the over-winter survival of the L35,10.
References
1. Charlier, J., Höglund, J., Morgan, E. R., Geldhof, P., Vercruysse, J., Claerebout, E. (2020). Biology and Epidemiology of Gastrointestinal Nematodes in Cattle. The Veterinary clinics of North America. Food animal practice, 36(1), 1–15.
2. Charlier, J., Claerebout, E., Vercruysse, J. (2016). Gastrointestinal Nematode Infections in Adult Dairy Cattle. Reference Module in Food Science, Elsevier.
3. Deplazes, P., Eckert, J., Mathis, A, von Samson-Himmelstjerna, G. and Zahner, H. (2016): Parasitology in Veterinary Medicine. Wageningen Academic publisers, The Netherlands.
4. Coles G. C. (2002). Sustainable use of anthelmintics in grazing animals. The Veterinary Record, 151(6), 165–169.
5. Zajac, A.M., Garza, J. (2020). Biology, Epidemiology, and Control of Gastrointestinal Nematodes of Small Ruminants. Veterinary Clinics of North America: Food Animal Practice, 36 (1), 73-87.
6. Craig T. M. (2018). Gastrointestinal Nematodes, Diagnosis and Control. The Veterinary clinics of North America. Food animal practice, 34(1), 185–199.
7. Rossanigo, C. E., Gruner, L. (1995). Moisture and temperature requirements in faeces for the development of free-living stages of gastrointestinal nematodes of sheep, cattle and deer. Journal of helminthology, 69(4), 357–362.
8. Hungerford, T.G. (1990). Diseases of Livestock. MacGraw-Hill Medical.
9. Dilrukshi Herath, H.M.P.; Taki, A.C.; Sleebs, B.E.; Hofmann, A., Nguyen, N.; Preston, S., Davis, R.H.; Jabbar, A., Gasser, R.B. (2021). Advances in the discovery and development of anthelmintics by harnessing natural product scaffolds. Advances in Parasitology (Eds. Rollinson, D; Russell J.). Academic Press, 111: 203-251.
10. Morgan, E. R., van Dijk, J. (2012). Climate and the epidemiology of gastrointestinal nematode infections of sheep in Europe. Veterinary parasitology, 189(1), 8–14.
About the author
Damien Achard (Ruminants Global Technical Manager)
Seasoned veterinarian, graduated from Ecole Nationale Vétérinaire de Nantes (France). After three years as a practitioner in central France, he pursued specialization in large animal internal medicine, completing an ACVIM residency and a Master of Sciences at the University of Montréal (2010-2014). Joining Semex Alliance as Health Manager for an IVF unit (2015-2016), he then transitioned to Ceva in 2016 as a Ruminants Global Technical Manager. Dr. Achard is an accomplished researcher, publishing on topics like downer cows, calf pneumonia or cryptosporidiosis and their associated therapies, and rational use of anthelmintics in ruminants. His ResearchGate profile (https://www.researchgate.net/profile/Damien-Achard/research) highlights his significant contributions to the veterinary field.
Explore author’s articlesAbout the author
Pablo Díaz is a Lecturer at the Universidade de Santiago de Compostela, Spain. He graduated and earned his PhD in Veterinary Science from the Universidade de Santiago de Compostela (1999 and 2006). His research is focused on the epidemiology, diagnosis, control and prevention of parasitic infections of wild and domestic animals. His research career comprises 104 papers in JCR-indexed journals (H-index of 21), including 63 Q1 publications, more than 40 articles in professional magazines as well as more than 240 communications to both National and International Scientific Conferences.
About the author
Susana Remesar (Assistant professor at USC)
Susana Remesar Alonso is an assistant professor at the Universidade de Santiago de Compostela, Spain. She completed her studies in Veterinary Medicine in 2015 and her PhD in 2019 at the Department of Animal Health of the University of Santiago de Compostela. Her research focuses on the parasitic diseases of animals, especially the study of ticks, their phenology, and tick-borne pathogens. She currently publishes the blog “Vetgraph: Medical & Veterinary Illustration” including illustrations about veterinary medicine, mainly related to parasites.

%20(1).webp)


Leave your comments here