Scours also known as neonatal diarrhoea (ND) is a gastrointestinal disease affecting calves during the first weeks of their lives and the most common cause of illness in both dairy and beef calves. The disease can be economically devastating to producers due to high mortality, impaired growth of affected animals and, later on, reduced milk production in the first lactation1–3. ND is also a significant welfare issue with affected calves displaying sickness behaviour such as increased lying time4 and decreased milk consumption5.
Scours can be caused by parasites, bacteria or viruses alone or in combination
Various enteric pathogens including viruses, bacteria and parasites can cause scours (Table 1) with rotavirus group A, coronavirus, Cryptosporidium parvum and bacterial pathogens (enterotoxigenic Escherichia coli F5 and Salmonella spp.) being the ones the most frequently incriminated in ill calves6–8. Co-infection, that is the detection within the same calf of more than one pathogen, is also frequently observed.
Table 1. Pathogens associated with neonatal diarrhoea (ND) in calves. The most frequently isolated enteric pathogens are listed in blue.
|
Viruses |
Bacteria |
Parasites |
|
Bovine rotavirus (group A) |
Enterotoxigenic Escherichia coli (F5/F41) |
Cryptosporidium parvum |
|
Bovine coronavirus |
Attaching and effacing E.coli |
Giardia duodenalis |
|
Bovine viral diarrhoea virus (BVDV) |
Extraintestinal pathogenic E.coli |
|
|
Bovine norovirus |
Salmonella spp. |
|
|
Nebovirus |
Clostridium perfringens |
|
|
Bovine enterovirus |
||
|
Bovine torovirus |
||
|
Bovine astrovirus |
||
|
Bovine parvovirus |
||
Scours in calves: when does it occur?
The occurrence of the various agents responsible for calf scours varies with the age of the calf. This can be useful in establishing the likelihood that a particular agent is involved (figure 1). For example, F5 E. coli typically causes diarrhoea in calves less than 6-days old, whereas Cryptosporidium is not detectable in the faeces of calves before 3-5 days old.
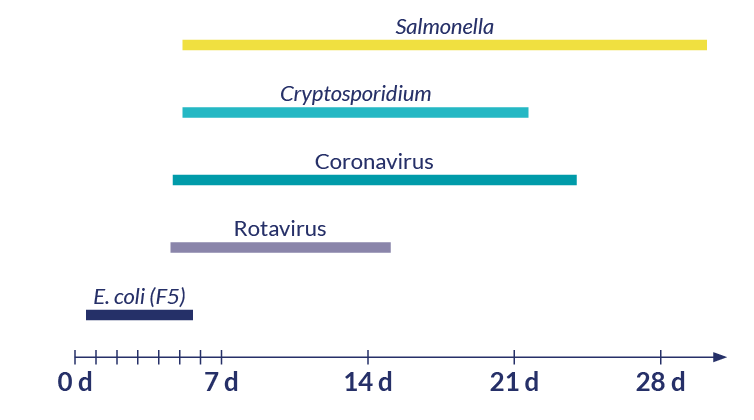
Figure 1. Timeline showing the average age of occurrence for scours in calves according to the causative agent.
Scours in calves: what are the “pathophysiological” consequences?
Whatever the pathogen(s) behind the clinical illness, affected calves excrete watery faeces with increased frequency. Without any treatment, the loss of liquids leads to a progressive dehydration, the loss of electrolytes, metabolic acidosis and possibly death within 2 days (figure 2)9.
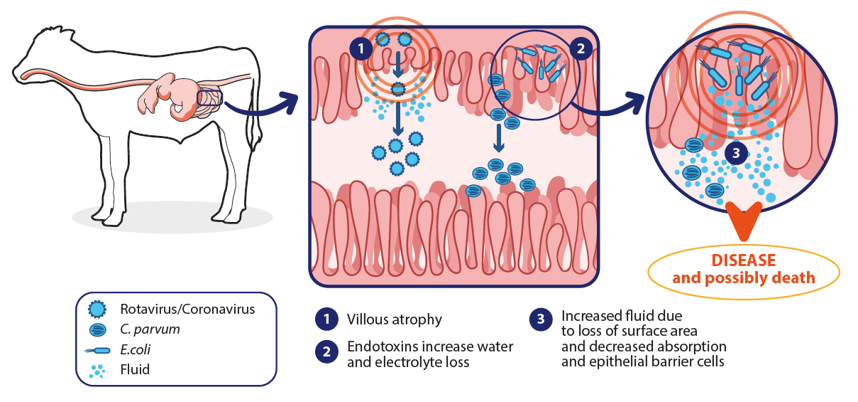
Figure 2. Pathophysiological consequences of scour. Adapted from Carter et al., 20219.
What are the characteristics of the major scours pathogens?
Rotavirus
Rotavirus (RVA) causes mild to severe diarrhoea in calves younger than 3 weeks old. The transmission occurs by the faecal-oral route. Infected animals shed around 1011 viral particles/gram of faeces. The virions are highly resistant to chemical and physical inactivation, remaining infectious in humid environments up to 4 months. Rotaviruses replicate in the mature enterocytes of the small intestine. The tips of the villi undergo cell lysis, resulting in villous blunting and ischemia, causing maldigested, malabsorptive and secretory diarrhoea10–12.
Coronavirus
Bovine coronavirus (CoV) causes pneumonia and diarrhoea in calves and adult cattle (winter dysentery). Calves become infected early after birth from faecal contamination of carrier dams and are susceptible between 1 day and 1 month of life. Infected calves shed high ~1 billion virions/g of faeces, remaining infectious only for up to 3 days in the presence of organic material unless faeces remain frozen. Its stability in frozen faeces and the aerosol transmission explains its prevalence in the winter months. CoV diarrhoea is prevalent in artificially reared dairy calves. Scour due to bovine coronavirus is usually more severe than that of rotavirus, resulting in muco-hemorrhagic enterocolitis (figure 3). Virus replication in the intestinal crypts leads to destruction of the absorptive intestinal villus epithelial cells in the entire small intestine, and eventually spreads throughout the large intestine, causing atrophy of colonic ridges and malabsorptive diarrhoea. Undigested nutrients are fermented in the large intestine, promoting bacterial overgrowth and production of organic acids, especially D-lactate, contributing to electrolyte losses and leading to dehydration and metabolic acidosis. The severity of the disease is increased and the incubation period is shortened in younger calves, especially those with inadequate passive transfer of immunity. Most calves recover, but some may develop pyrexia, recumbency, cardiovascular collapse, coma and death. Some CoV strains behave as a pneumoenteric pathogen, also replicating in the upper respiratory tract. Infected calves may develop both diarrhoea and respiratory disease15–17.

Figure 3. Calf with bovine coronavirus scour14.
Escherichia coli
E.coli is a gram-negative bacterium. Diarrheagenic E. coli are now broadly classified according to virulence mechanisms. Enterotoxigenic strains of E. coli (ETEC) bearing different pili (F5, F17 and CS31A) bind to the intestinal epithelium without causing apparent damage to enterocytes and produce a thermostable toxin (ST) that causes secretory diarrhoea mostly in the first week of life. Other pathotypes include enterohemorrhagic (EHEC) producing hemolisin, shiga-toxin (STEC) and attaching-effacing (AEEC) E. coli that are usually associated with dysentery in calves between 8 to 21 days of age. AEEC allows the attachment of the bacteria to the apical cell membrane of the enterocyte causing a lesion characterised by degeneration and exfoliation of the microvilli in the small and large intestine of calves between 1 and 5 weeks of age. The infection causes enterocolitis, diarrhoea and dysentery. Other E.coli strains invade the bloodstream causing septicemia 18-22.
Salmonella spp.
Salmonella is a genus comprising gram-negative bacteria belonging to the family Enterobacteriaceae. Salmonella enterica serovar Dublin is host-specific for cattle while Salmonella enterica serovar Typhimurium is pathogenic for cattle and other species. Calves from two weeks up to 3 months old may become clinically infected. During an outbreak, calves may show diarrhoea with or without blood and mucus, fever, depression, respiratory signs, septicemia, and death. Macroscopic observation shows jaundice, pseudomembranes on the small intestinal mucosa and enlargement of the mesenteric lymph nodes. After an outbreak is controlled, several animals may remain as carriers. Cows become carriers of S. Dublin, with intermittent shedding, at higher levels in the peripartum period. In dairy farms, especially those with large numbers of cattle, Salmonellosis may become endemic. Salmonella infections usually occur via the fecal-oral route23.
Cryptosporidium parvum
Cryptosporidium parvum is a protozoan frequently isolated in calves with diarrhoea and that infects the distal small intestine causing a mild to severe diarrhoea. In severe cases faeces can contain mucus, blood, undigested milk, or bile. C. parvum is transmitted via fecal-oral ingestion of oocysts that are resistant to most disinfectants and can survive for several months in cool and moist conditions. Susceptibility windows in calves go from one to four weeks of age. Affected animals frequently need oral or parenteral fluids to restore their hydration and metabolic disturbances. Halofuginone and paromomycin are reported to be effective treatments.1,24–27
References
1. De Graaf, D. C., Vanopdenbosch, E., Ortega-Mora, L. M., Abbassi, H. & Peeters, J. E. A review of the importance of cryptosporidiosis in farm animals. Int J Parasitol 29, (1999).
2. Häsler, B., Howe, K. S., Presi, P. & Stärk, K. D. C. An economic model to evaluate the mitigation programme for bovine viral diarrhoea in Switzerland. Prev Vet Med 106, (2012).
3. Abuelo, A., Cullens, F. & Brester, J. L. Effect of preweaning disease on the reproductive performance and first-lactation milk production of heifers in a large dairy herd. J Dairy Sci 104, (2021).
4. Goharshahi, M. et al. Monitoring selected behaviors of calves by use of an ear-attached accelerometer for detecting early indicators of diarrhea. J Dairy Sci 104, (2021).
5. Sutherland, M. A., Lowe, G. L., Huddart, F. J., Waas, J. R. & Stewart, M. Measurement of dairy calf behavior prior to onset of clinical disease and in response to disbudding using automated calf feeders and accelerometers. J Dairy Sci 101, (2018).
6. Galma Boneya Arero. Review on Common Infectious Diseases of Neonatal Calves. Journal of Veterinary Science and Research (2021)
7. Ryu, J. H., Shin, S. U. & Choi, K. S. Molecular surveillance of viral pathogens associated with diarrhea in pre-weaned Korean native calves. Trop Anim Health Prod 52, (2020).
8. Cho, Y. il & Yoon, K. J. An overview of calf diarrhea - infectious etiology, diagnosis, and intervention. J Vet Sci 15, (2014).
9. Carter, H. S. M., Renaud, D. L., Steele, M. A., Fischer-Tlustos, A. J. & Costa, J. H. C. A narrative review on the unexplored potential of colostrum as a preventative treatment and therapy for diarrhea in neonatal dairy calves. Animals vol. 11.
10. Parreño, V. et al. Milk supplemented with immune colostrum: Protection against rotavirus diarrhea and modulatory effect on the systemic and mucosal antibody responses in calves experimentally challenged with bovine rotavirus. Vet Immunol Immunopathol 136, 12–27 (2010).
11. Badaracco, A., Garaicoeachea, L., Mathijnssens, J. & Parreño, V. Epidemiology of rotavirus infection in cattle, small ruminants and horses. Rotavirus Infections: Epidemiology, Clinical Characteristics and Treatment Options (2014).
12. Parreño, V. et al. Modulation by colostrum-acquired maternal antibodies of systemic and mucosal antibody responses to rotavirus in calves experimentally challenged with bovine rotavirus. Vet Immunol Immunopathol 100, (2004).
13. Bok, M. et al. Passive immunity to control Bovine coronavirus diarrhea in a dairy herd in Argentina. Rev Argent Microbiol 50, (2018).
14. Bok, M. et al. Molecular and antigenic characterization of bovine Coronavirus circulating in Argentinean cattle during 1994-2010. Vet Microbiol 181, (2015).
15. Castells, M. et al. Bovine coronavirus in Uruguay: genetic diversity, risk factors and transboundary introductions from neighboring countries. Arch Virol 164, (2019).
16. Vlasova, A. N. & Saif, L. J. Bovine Coronavirus and the Associated Diseases. Frontiers in Veterinary Science vol. 8
17. Neonatal calf diarrhea induced by rotavirus and coronavirus: a review. International Journal of Biosciences (IJB) 6, (2015).
18. Khawaskar, D. P. et al. Pathotyping and antimicrobial susceptibility testing of Escherichia coli isolates from neonatal calves. Vet Res Commun 46, (2022).
19. Umpiérrez, A. et al. Prevalence of Escherichia coli adhesion-related genes in neonatal calf diarrhea in Uruguay. J Infect Dev Ctries 10, (2016).
20. Louge Uriarte, E. L. et al. Molecular characterization of multidrug-resistant Escherichia coli of the phylogroups A and C in dairy calves with meningitis and septicemia. Microb Pathog 163, (2022).
21. Prieto, A. et al. Antimicrobial Susceptibility of Enterotoxigenic Escherichia coli from Diarrhoeic Neonatal Calves in Spain. Animals 12, (2022).
22. Umpiérrez, A. et al. Virulence genes of Escherichia coli in diarrheic and healthy calves. Rev Argent Microbiol 53, (2021).
23. Smith, D. R. Field Disease Diagnostic Investigation of Neonatal Calf Diarrhea. Veterinary Clinics of North America - Food Animal Practice vol. 28 (2012).
24. Conrady, B., Brunauer, M. & Roch, F. F. Cryptosporidium spp. Infections in combination with other enteric pathogens in the global calf population. Animals vol. 11 (2021).
25. Arsenopoulos, K., Theodoridis, A. & Papadopoulos, E. Effect of colostrum quantity and quality on neonatal calf diarrhoea due to Cryptosporidium spp. infection. Comp Immunol Microbiol Infect Dis 53, (2017).
26. Garro, C. J. et al. Occurrence of Cryptosporidium and other enteropathogens and their association with diarrhea in dairy calves of Buenos Aires province, Argentina. Vet Parasitol Reg Stud Reports 24, (2021).
27. Brunauer, M., Roch, F. F. & Conrady, B. Prevalence of worldwide neonatal calf diarrhoea caused by bovine rotavirus in combination with bovine coronavirus, escherichia coli k99 and cryptosporidium spp.: A meta-analysis. Animals vol. 11 (2021).
About the author
Damien Achard (Ruminants Global Technical Manager)
Seasoned veterinarian, graduated from Ecole Nationale Vétérinaire de Nantes (France). After three years as a practitioner in central France, he pursued specialization in large animal internal medicine, completing an ACVIM residency and a Master of Sciences at the University of Montréal (2010-2014). Joining Semex Alliance as Health Manager for an IVF unit (2015-2016), he then transitioned to Ceva in 2016 as a Ruminants Global Technical Manager. Dr. Achard is an accomplished researcher, publishing on topics like downer cows, calf pneumonia or cryptosporidiosis and their associated therapies, and rational use of anthelmintics in ruminants. His ResearchGate profile (https://www.researchgate.net/profile/Damien-Achard/research) highlights his significant contributions to the veterinary field.
Explore author’s articlesAbout the author
Viviana Parreño (Biochemist - PhD Veterinary immunology and virology)
Dr Parreño is an expert in viral pathogens associated with neonatal diarrhoea in human and animal species (Rotavirus, Norovirus, Coronavirus), her team conduct epidemiological surveys, diagnosis, and molecular characterisation of enteropathogens. She also works in the development of passive immune strategies based on chicken egg yolk IgY and llama-derived nanobodies (recombinant monoclonal nano antibodies). She worked as well in the development and testing of viral vaccines in animal models (mice, calves, gnotobiotic pigs) and conducted the development and statistical validation of a guinea pig model as an alternative method for vaccine potency testing. The method was adopted as the official control in Argentina of combined vaccines applied in cattle containing IBR, BVDV, PI3, RVA and CoV.




Leave your comments here