Q fever also named coxiellosis is an infectious disease caused by an obligatorily intracellular bacterium, Coxiella burnetii. Many animals species including dogs, cats, rabbits, rodents, birds and even some arthropods are susceptible to be infected by this bacterium. But ruminants (cattle, goats and sheeps) are the main reservoir of the bacteria which can also contaminate humans.
Techniques to assess the prevalence of Q fever in cattle
There are several ways to assess the prevalence of Q fever in cattle:
- It can be estimated at the herd level: what is the ratio of infected herds within a country or area? An infected herd being defined as a herd with at least one positive animal to Q fever.
- But the prevalence can also be assessed at animal level: what is the ratio of positive animals in a country or a zone?
The figures given by these two ways can differ slightly. In extreme cases, the prevalence at herd level can be 100%: all the herds studied are infected. But at animal level, the prevalence can be lower. For example, in a study carried out in Egypt in 2012-2013, all the farm tested were infected but only 13% of the animals were positive (Gwida et al., 2014).
There are two main ways to evaluate the positivity of animals and herds. They can be gathered in two families:
- Direct diagnosis: currently, direct diagnosis is carried out using molecular techniques and mainly rt-PCR or qPCR (real time Polymerase Chain Reaction or quantitative Polymerase Chain Reaction). These techniques can be used on several samples including feces, vaginal swab, placenta… and milk. This latest is of main interest as it includes bulk tank milk, which can be relevant for epidemiological studies in dairy cattle.
- Indirect diagnosis (serology): the technique used is mostly ELISA (Enzyme Linked ImmunoSorbent Assay) as it is reliable and cost effective. But some other Q fever tests can also be used as ImmunoFluorescence or Complement Fixation Test. All these techniques aim to detect specific antibodies against Coxiella burnetii and can be performed on blood or milk, including bulk tank milk.
In the case of epidemiological survey both family of tests are relevant, but neither is absolutely perfect:
- Direct diagnosis can underestimate the real prevalence because the shedding of Coxiella burnetii by the animals is intermittent.
- Indirect diagnosis can overestimate the real prevalence because antibodies can be found in blood or milk, even if the animals are no longer infected.

Prevalence of Q fever in cattle
With the exception of New-Zealand, Coxiella burnetii is present worldwide.
Several studies assessed the prevalence of Q fever in cattle in many countries, using serology or PCR. Depending on the study, the prevalence may vary slightly but the number of herds and animals affected is never negligible.
The tables and maps below summarize the prevalence of Q fever in cattle according to data published in scientific literature over the last 30 years. The first table (table 1) and map (figure 1) show the prevalence at animal level while Table 2 and Figure 2 consider it at herd level:
Table 1. Prevalence of Q fever in cattle at animal level
|
Country |
Number of animals tested |
Number of positive animals |
Prevalence |
|
|
Africa |
28403 |
6163 |
21,7% |
|
|
Cameroon |
14754 |
4628 |
31,4% |
|
|
Chad |
442 |
29 |
6,6% |
|
|
Egypt |
1294 |
180 |
13,9% |
|
|
Ethiopia |
422 |
37 |
8,8% |
|
|
Ghana |
166 |
30 |
18,1% |
|
|
Guinea |
463 |
95 |
20,5% |
|
|
Nigeria |
1013 |
327 |
32,3% |
|
|
Senegal |
196 |
8 |
4,1% |
|
|
South Africa |
9231 |
723 |
7,8% |
|
|
Togo |
242 |
36 |
14,9% |
|
|
Zimbabwe |
180 |
70 |
38,9% |
|
|
America |
3225 |
464 |
14,4% |
|
|
Canada |
75 |
18 |
24,0% |
|
|
Colombia |
482 |
110 |
22,8% |
|
|
Ecuador |
2668 |
336 |
12,6% |
|
|
Asia |
9943 |
1619 |
16,3% |
|
|
Bangladesh |
620 |
4 |
0,6% |
|
|
China |
1395 |
412 |
29,5% |
|
|
Iran |
497 |
69 |
13,9% |
|
|
Japan |
562 |
262 |
46,6% |
|
|
Korea |
3077 |
177 |
5,8% |
|
|
Lebanon |
865 |
86 |
9,9% |
|
|
Nepal |
162 |
2 |
1,2% |
|
|
Saudi Arabia |
518 |
169 |
32,6% |
|
|
Turkey |
1488 |
161 |
10,8% |
|
|
United Arab Emirates |
759 |
277 |
36,5% |
|
|
Europe |
65477 |
8324 |
12,7% |
|
|
Albania |
863 |
81 |
9,4% |
|
|
Bulgaria |
16728 |
1498 |
9,0% |
|
|
Cyprus |
75 |
18 |
24,0% |
|
|
Denmark |
2188 |
625 |
28,6% |
|
|
France |
95 |
23 |
24,2% |
|
|
Germany |
25093 |
3468 |
13,8% |
|
|
Hungary |
697 |
271 |
38.9% |
|
|
Ireland |
5182 |
321 |
6,2% |
|
|
Italy |
1361 |
323 |
23,7% |
|
|
Netherlands |
7792 |
743 |
9,5% |
|
|
Poland |
2973 |
707 |
23,8% |
|
|
Spain |
2071 |
229 |
11,1% |
|
|
Switzerland |
359 |
17 |
4,7% |
|
|
Oceania |
4086 |
10 |
0,2% |
|
|
Australia |
1905 |
10 |
0,5% |
|
|
New Zealand |
2181 |
0 |
0,0% |
|
|
Grand total |
111134 |
16580 |
14.9% |
|
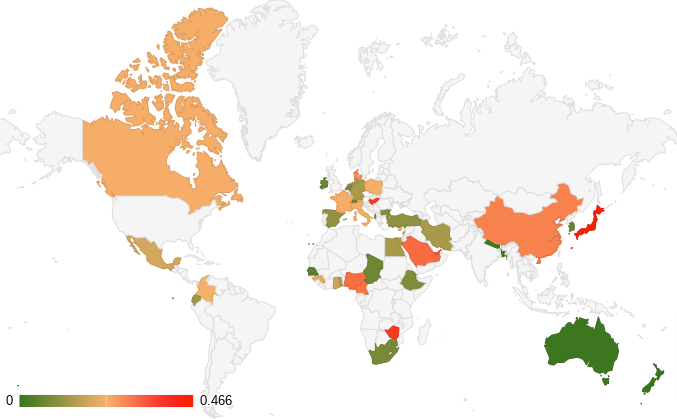
Figure 1. Map of the prevalence at animal level
Table 2. Prevalence of Q fever in cattle at herd level
|
Country |
Number of herds tested |
Number of Positive herds |
Global herd prevalence |
|
Africa |
182 |
119 |
65% |
|
Cameroon |
146 |
99 |
68% |
|
Egypt |
9 |
9 |
100% |
|
Nigeria |
27 |
11 |
41% |
|
America |
1779 |
1165 |
65% |
|
Canada |
222 |
83 |
37% |
|
Columbia |
11 |
5 |
45% |
|
Ecuador |
386 |
181 |
47% |
|
USA |
1160 |
896 |
77% |
|
Asia |
1617 |
324 |
20% |
|
China |
19 |
16 |
84% |
|
Iran |
642 |
70 |
11% |
|
Jordan |
78 |
55 |
71% |
|
Korea |
607 |
108 |
18% |
|
Lebanon |
173 |
53 |
31% |
|
Turkey |
98 |
22 |
22% |
|
Europe |
11166 |
4849 |
43% |
|
Belgium |
256 |
134 |
52% |
|
Croatia |
39 |
33 |
85% |
|
Czech Republic |
414 |
331 |
80% |
|
Denmark |
2476 |
956 |
39% |
|
France |
145 |
58 |
40% |
|
Germany |
173 |
108 |
62% |
|
Greece |
80 |
28 |
35% |
|
Hungary |
437 |
347 |
79% |
|
Ireland |
563 |
242 |
43% |
|
Italy |
1753 |
681 |
39% |
|
Latvia |
252 |
27 |
11% |
|
Netherlands |
991 |
519 |
52% |
|
Poland |
1171 |
323 |
28% |
|
Portugal |
90 |
26 |
29% |
|
Serbia |
72 |
42 |
58% |
|
Slovakia |
159 |
127 |
80% |
|
Slovenia |
48 |
28 |
58% |
|
Spain |
427 |
251 |
59% |
|
Switzerland |
872 |
344 |
39% |
|
United Kingdom |
748 |
244 |
33% |
|
Oceania |
49 |
6 |
12% |
|
Australia |
49 |
6 |
12% |
|
Grand Total |
14793 |
6463 |
44% |
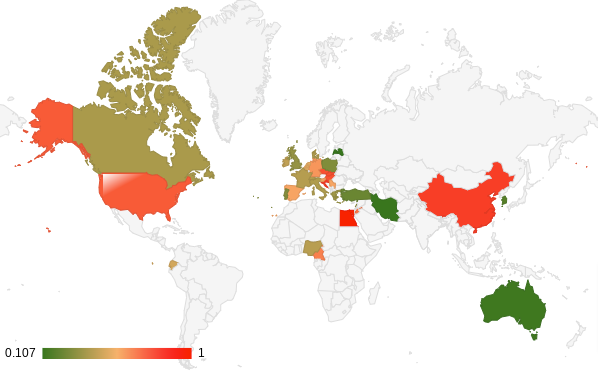
Figure 2. Map of the prevalence at herd level
These data highlight the high prevalence of Q fever in cattle. At animal level, it ranges from 0% in New-Zealand to 46,62% in Japan. On the other hand, the herd prevalence is very high in most of the countries. The study carried out in Egypt found that all the herds were infected. Even if it is an extreme case, globally the data available for 34 countries shows that for 27 of them, the herd prevalence is above 30%, and for 16 of them, above 50% meaning that at least 1 cattle herd out of 2 is contaminated by Q fever.
Key Message
Q fever is therefore a ubiquitous disease present in a large part of cattle herd around the world. But most of the infected farms are not aware about their status regarding Q fever. A better diagnosis and consequently a better control of the disease would lead to an improvement of cattle welfare and farm productivity and also to a lower risk of human contamination as Q fever is zoonotic disease.
About the author
Philippe Gisbert (Ruminants Global Technical Manager)
Philippe Gisbert started his career in 1994 as a Vet practitioner working with companion and farm animals for over 9 years. He then became Health Affairs Manager for Group Agena (artificial insemination company). In 2008 he joined Eurofins – Laboratoire Coeur de France as Animal Health Unit Manager where he worked for 7 years until he joined Ceva France as Technical Manager Ruminants (Infectiology, Vaccines and Diagnostic). Since 2020 he is Global Technical Manager for Biologicals, Udder Health and Antiinflammatories. He is a member of SIMV diagnostic and anti-infective technical groups and has integrated different working groups of ANSES and UNCEIA related to epidemiology, antibiotic resistance and reproduction in livestock.
Explore author’s articlesFrequently Asked Questions
-
What are the symptoms of Q fever in cattle?
Q fever is essentially a reproductive disease in cattle. The clinical signs are abortions, weak newborns, stillbirths, placental retention, metritis/endometritis and more generally fertility disorders. -
What is the best treatment for Q fever?
As, in ruminants, medical treatment (antibiotics) are not efficient to treat the disease, the best way to control Q fever is to prevent it by biosecurity measures and vaccination. However, in an infected herd, vaccination has also been shown to reduce the clinical impact of the disease and to reduce the shedding of the bacteria by the animals. -
Is there a vaccine for Q fever in cattle?
Yes. Ceva developed a unique phase I vaccine against Q Fever. The commercially available vaccine reduces both the clinical signs of the disease, such as abortions, and the shedding of the causative bacteria. -
How is Q fever diagnosed?
QTest is a new way to easily sample, store, and ship samples to perform Q fever PCR analysis on bulk tank milk. QTest is a tool of choice for Q fever testing as it allows a sample of bulk tank milk to be safely stored for several days, even at high and low temperatures, and to provide a reliable diagnosis. -
How do you prevent Q fever in cows?
The prevention of Q fever in ruminant herds involves non-medical measures (biosecurity) and animal vaccination. The commercially available vaccine reduces both the clinical signs of the disease, such as abortions, and the shedding of the causative bacteria.


Leave your comments here