Bovine respiratory disease (BRD) is a major infectious disease of calves affecting their health, decreasing their growth, and impacting the profitability of cattle herds across the globe. Key bacteria and viruses are known to cause BRD such as Mannheimia haemolytica or Bovine Respiratory Syncytial Virus. Among these important pathogens, Mycoplasma bovis (M. bovis) hold a special place. Indeed, this bacterium possess unique biological characteristics that explain why M. bovis is so successful to evade the host immune response and is a dominant pathogen in many cattle herds responsible for arthritis, otitis and most importantly, acute and chronic cases of pneumonia.
M. bovis is a unique microorganism
M. bovis is a bacterium of the class Mollicutes, the smallest organisms that can self-replicate without hosts1.
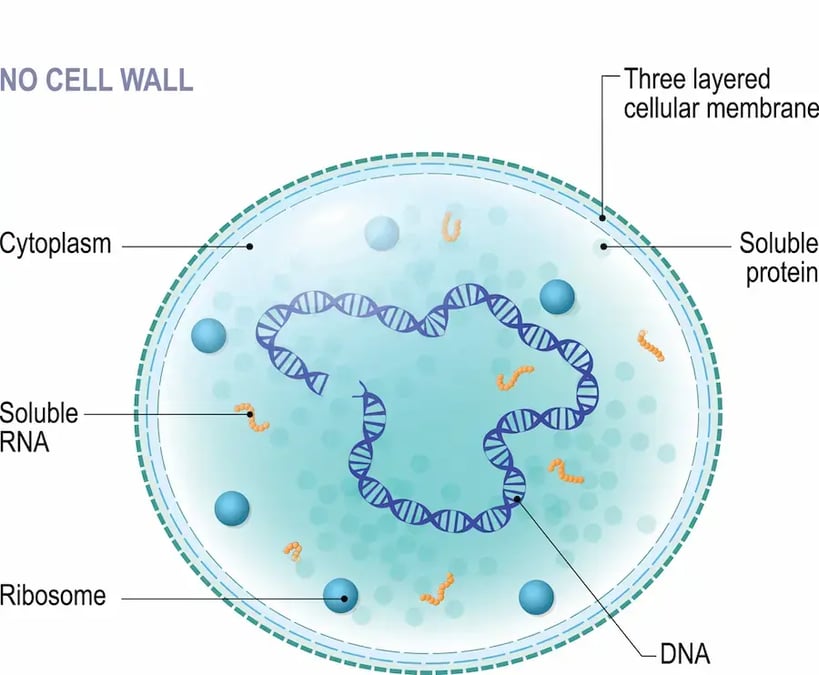
Its lack of cell wall which means that it does not stain with Gram and that it remains unaffected by antibiotics of the beta-lactam or cephalosporin families which target bacteria with a cell wall. Its small genome size prevents the synthesizing of amino acids and some fatty acids. Therefore, during in vitro culture, it has complex nutritional requirements1, 2, 3. For this reason, its transport and its culture in lab are difficult, which leads to underdiagnoses and underestimation of its role in BRD4.
However, it has great pathogenic capability. M. bovis is able to penetrate, survive, prevent proliferate and suppress the functions of epithelial cells, blood mononuclear cells, alveolar macrophages or erythrocytes. Likewise, it is capable of evading macrophage phagocytosis and modulating cytokine secretion. It can also produce biofilms and vary its surface antigens by avoiding the action of specific antibodies, thus evading the host's immune response5, 6.
M. bovis is the most relevant species for young calves
Several mycoplasma species are occasionally isolated from sick cattle, but only M. bovis is recognized as a primary cause of BRD and is considered one of the most common bacterial pathogen involved in BRD worldwide3, 7.
Its health, economic and animal welfare importance is due to its high contagiousness, up to 100% in large feedlots, its limited response to antibiotic treatments and the ease with which infections become chronic3, 8.
In addition to pneumonia, M. bovis causes may other diseases in calves. For example polyarthritis, usually in stifle and carpus joints; otitis media; decubitus abscesses or endocarditis, among others9, 10, 11, 12, 13.
M. bovis is a major cause of calf pneumonia in dairys, feedlots and veal calf operations

The prevalence of M. bovis infection varied with geographic location and type of production systems. Its presence is typically high in cattle operations where mixing animals from different sources is frequent (feedlots, veal calves) with 50 % of sick animals M. bovis positive during BRD outbreaks. Dairy farms can also be affected, as illustrated recently by a polish study that found a PCR detection rate of nearly 30% out of 74 dairy herds14.
When the source of contagion is the dam, the infection appears in the first month of life and persists and spreads through the rearing pens15. Subsequently, either through chronic calves or asymptomatic carrier animals, the disease reaches veal calf units and feedlots, where, taking advantage of the high stocking density and sometimes poor ventilation, it spreads very quickly.
Coinfection with virus, especially BVD, and Pasteurella, especially M. haemolytica is very common16, 17. In these coinfection, M. bovis does not seem to be a secondary agent, but rather a primary agent that causes the overgrowth of M. haemolytica18.
Traditionally, M. bovis has been associated with chronic cases of BRD that do not respond to treatment19. However recent studies have shown that M. bovis can be directly responsible for acute pneumonia but also predisposes to the M. haemolytica infections found in most BRD outbreaks9, 18, 21
M. bovis is highly contagious
Transmission of M. bovis can occur from the mother through contaminated colostrum, milk, nasal or vaginal secretions. In dairy calves, the use of unpasteurized waste milk from asymptomatic carriers may be the source of infection22.
When infected calves, asymptomatic or not, arrive at markets, rearing centers or feedlots, they quickly spread the disease through aerosols, especially in the presence of high densities, poor ventilation, cold or humid environments. Poorly disinfected feeding equipment can also be a source of contagion13.
Chronic calves spread a large number of germs in the air. Therefore, isolation in infirmaries and good ventilation and hygiene are essential to prevent disease22.
Conclusion
M. bovis is not only the most important species in its genus, but also one of the main causative agent of BRD in calves. M. bovis is a truly unique pathogen with high contagious capacity, an ability to evade the immune system, the possibility of producing acute and chronic infections and its ability to cause pneumonia, arthritis, and otitis. All this, together with the limited availability of vaccines and its high level of resistance to antimicrobials, means that producers and veterinarians must make it one of their focus to ensure the health and welfare of young calves.
References
1. Razin, S., Yogev, D., Naot, Y. 1998. Molecular biology and pathogenicity of mycoplasmas. Microbiology and Molecular Biology Reviews: MMBR, 62(4), 1094-1156.
2. Parte A, Krieg NR, Ludwig W. Bergey’s Manual of Systematic Bacteriology. New York, NY: Springer New York; 2011.
3. Parker, A. M., Sheehy, P. A., Hazelton, M. S., Bosward, K. L., and House, J. K. (2018). A review of mycoplasma diagnostics in cattle. J. Vet. Intern. Med. 32, 1241–1252.
4. Prakash, O., Parmar, M., Vaijanapurkar, M., Rale, V., and Shouche, Y. S. (2021). Recent trend, biases and limitations of cultivation-based diversity studies of microbes. FEMS Microbiol. Lett. 368, fnab118.
5. Maunsell, F.P., Chase, C., 2019. Mycoplasma bovis: Interactions with the immune system and failure to generate an effective immune response. The Veterinary clinics of North America. Food animal practice 35, 471-483.
6. Perez-Casal 2020 Pathogenesis and Virulence of Mycoplasma bovis. Vet Clin Food Anim 36 (2020) 269–278
7. Rosenbusch RF. Mycoplasmas in bovine respiratory disease. In: Anderson DE, Rings DM, editors. Current veterinary therapy: food animal practice. 5th edition. St Louis (MO): Saunders-Elsevier; 2009. p. 192–4.
8. Radaelli E, Luini M, Loria GR, Nicholas RA, Scanziani E. Bacteriological, serological, pathological and immunohistochemical studies of Mycoplasma bovis respiratory infection in veal calves and adult cattle at slaughter. Res Vet Sci. 2008;85(2):282-90
9. Timsit E, Workentine M, van der Meer F, et al. Distinct bacterial metacommunities inhabit the upper and lower respiratory tracts of healthy feedlot cattle and those diagnosed with bronchopneumonia. Vet Microbiol 2018;221:105–13.
10. Constant, C., Nichols, S., Desrochers, A., Babkine, M., Fecteau, G., Lardé, H., Fairbrother, J., Francoz, D., 2018. Clinical findings and diagnostic test results for calves with septic arthritis: 64 cases (2009–2014). Journal of the American Veterinary Medical Association 252, 995-1005.
11. Lamm, C.G., Munson, L., Thurmond, M.C., Barr, B.C., George, L.W., 2004. Mycoplasma otitis in California calves. Journal of Veterinary Diagnostic Investigation 16, 397-402.
12. Kinde, H., Daft, B.M., Walker, R.L., Charlton, B.R., Petty, R., 1993. Mycoplasma bovis associated with decubital abscesses in Holstein calves. Journal of Veterinary Diagnostic Investigation 5, 194-197.
13. Kanda, T., Tanaka, S., Suwanruengsri, M., Sukmawinata, E., Uemura, R., Yamaguchi, R., Sueyoshi, M., 2019. Bovine endocarditis associated with Mycoplasma bovis. Journal of Comparative Pathology 171, 53-58.
14. Lachowicz-Wolak, A.; Klimowicz-Bodys, M.D.; Płoneczka-Janeczko, K.; Bykowy, M.; Siedlecka, M.; Cinciała, J.; Rypuła, K. The Prevalence, Coexistence, and Correlations between Seven Pathogens Detected by a PCR Method from South-Western Poland Dairy Cattle Suffering from Bovine Respiratory Disease. Microorganisms 2022, 10, 1487.
15. Wikse, S.E., 1985. Feedlot cattle pneumonia. The veterinary clinics of North America. Food Animal Practice 1, 289–310.
16. Thomas, A., Ball, H., Dizier, I., Trolin, A., Bell, C., Mainil, J., Linden, A., 2002. Isolation of mycoplasma species from the lower respiratory tract of healthy cattle and cattle with respiratory disease in Belgium. The Veterinary Record 151, 472-476.
17. Haines, D.M., Martin, K.M., Clark, E.G., Jim, G.K., Janzen, E.D., 2001. The immunohistochemical detection of Mycoplasma bovis and bovine viral diarrhea virus in tissues of feedlot cattle with chronic, unresponsive respiratory disease and/or arthritis. The Canadian veterinary journal = La revue veterinaire canadienne 42, 857-860.
18. Valeris-Chacin R, Powledge S, McAtee T, Morley PS and Richeson J (2022) Mycoplasma bovis is associated with Mannheimia haemolytica during acute bovine respiratory disease in feedlot cattle. Front. Microbiol. 13:946792.
19. Caswell, J.L.; Archambault, M. Mycoplasma bovis pneumonia in cattle. Anim. Health Res. Rev. 2007, 8, 161–186.
20. Nicholas, R.A.J., Ayling, R.D., 2003. Mycoplasma bovis: disease, diagnosis, and control. Research in Veterinary Science 74, 105–112.
21. Arcangioli, M.-A.; Duet, A.; Meyer, G.; Dernburg, A.; Bézille, P.; Poumarat, F.; Le Grand, D. The role of Mycoplasma bovis in bovine respiratory disease outbreaks in veal calf feedlots. Vet. J. Lond. Engl. 1997 2008, 177, 89–93.
22. Maunsell, F.P.; Woolums, A.R.; Francoz, D.; Rosenbusch, R.F.; Step, D.L.; Wilson, D.J.; Janzen, E.D. Mycoplasma bovis infections in cattle. J. Vet. Intern. Med. 2011, 25, 772–783.
About the author
Damien Achard (Ruminants Global Technical Manager)
Seasoned veterinarian, graduated from Ecole Nationale Vétérinaire de Nantes (France). After three years as a practitioner in central France, he pursued specialization in large animal internal medicine, completing an ACVIM residency and a Master of Sciences at the University of Montréal (2010-2014). Joining Semex Alliance as Health Manager for an IVF unit (2015-2016), he then transitioned to Ceva in 2016 as a Ruminants Global Technical Manager. Dr. Achard is an accomplished researcher, publishing on topics like downer cows, calf pneumonia or cryptosporidiosis and their associated therapies, and rational use of anthelmintics in ruminants. His ResearchGate profile (https://www.researchgate.net/profile/Damien-Achard/research) highlights his significant contributions to the veterinary field.
Explore author’s articles



Leave your comments here