Calf pneumonia is one of the most important condition impacting the global beef and dairy industry. It is the second most common disease after neonatal calf diarrhea with morbidities between 18% to 24%1–4. It is a multifaceted problem with host, management, viral and environmental factors combining to predispose cattle to respiratory disease. Calf pneumonia is a major cause of economic loss for the cattle industry, due to treatment costs, reduced performance (loss of weight or absence of weight gain, lighter carcass at slaughter or reduced milk production), and animal death1,3.
Calf pneumonia is commonly caused by bacteria
In this article, we will focus on the bacterial pathogens associated with calf pneumonia, as pneumonia caused by bacterial pathogens represents the most significant cause of morbidity and mortality of bovine respiratory disease (BRD)5. Major bacterial pathogens that cause bronchopneumonia in beef, dairy, and veal calves include Mannheimia haemolytica, Pasteurella multocida, Histophilus somni, and Mycoplasma bovis6. These bacteria are opportunistic pathogens as they can be isolated from the upper and lower airways of healthy cattle7. It is generally accepted that it is only when predisposing factors reduce respiratory defenses that these bacterial pathogens can colonize and proliferate in the lower airways (i.e. bronchiole and alveoli) leading to bronchopneumonia (Figure 1). Interestingly, numerous studies have reported a high frequency of co-infections or confirmed that the presence of multiple pathogens is more often associated with illness than mono-infections8.
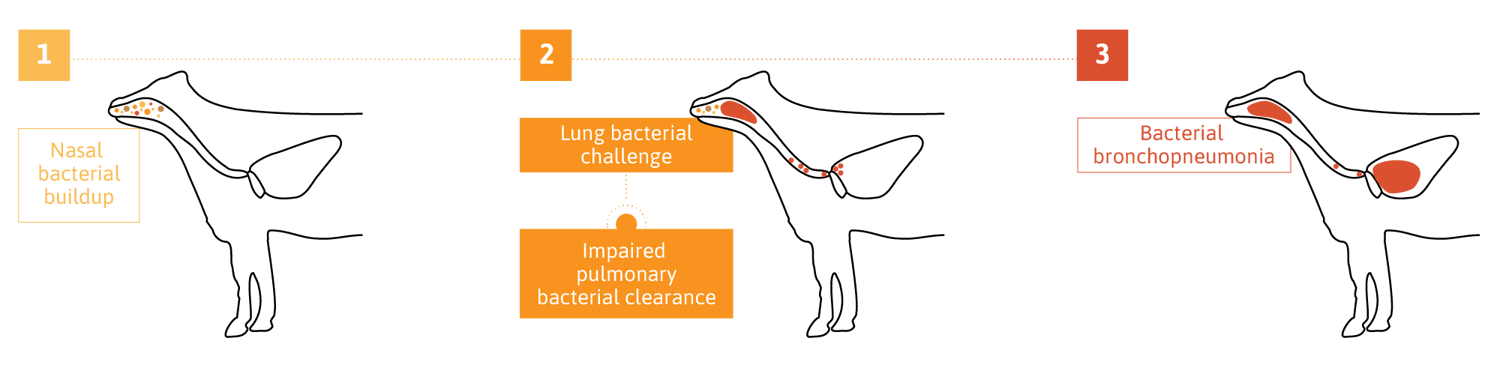
Figure 1. Cascade of events leading to bacterial bronchopneumonia
Mannheimia haemolytica
Mannheimia haemolytica is generally considered the most common bacterium isolated from sick cattle but the proportion of bronchopneumonia cases attributable to Pasteurella multocida appears to be increasing. Under normal conditions, M. haemolytica remains confined to the upper respiratory tract, in particular the tonsillar crypts. After stress or viral infection, the replication rate of M. haemolytica in the upper respiratory tract increases rapidly. The increased bacterial growth rate in the upper respiratory tract is followed by inhalation and colonization of the lungs. M. haemolytica causes severe, acute, hemorrhagic fibronecrotic pneumonia. Grossly, there are extensive reddish-black to grayish-brown cranioventral regions of consolidation with gelatinous thickening of interlobular septa and fibrinous pleuritis. There are extensive thromboses, foci of lung necrosis, and limited evidence of bronchitis and bronchiolitis. During the exponential growth of the bacteria in the lungs, virulence factors are produced by M. haemolytica, in particular an exotoxin that has been referred to as leukotoxin and an endotoxin described as lipopolysaccharide (LPS). The secretion of leukotoxin and the release of LPS together play a central role in the migration of neutrophils into the lungs, and these immune cells are largely responsible for the excessive pulmonary inflammation, and tissue damage associated with calf pneumonia (Figure 2).
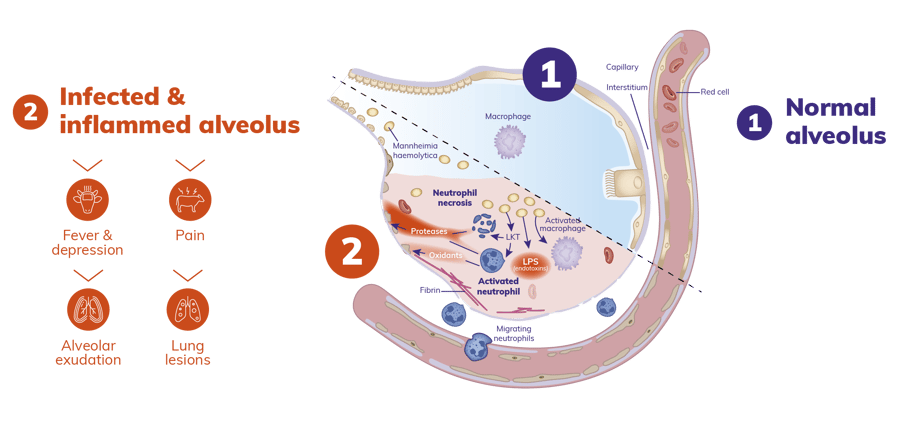
Figure 2. Pathogenesis of calf pneumonia. Adapted with authorization (9).
Pasteurella multocida
Pasteurella multocida is also an important cause of bacterial pneumonia. The most recent epidemiological reports available in dairy cattle, beef herds and veal calves in Europe suggest an increase in its detection10-12. Co-infections of P. multocida with other respiratory pathogens in diseased animals are also commonly recorded.
The pathogenesis of pneumonia caused by P. multocida is poorly understood. P. multocida is associated with fibrinous to fibrinopurulent bronchopneumonia, small amounts of fibrin exudation, some thromboses, limited lung necrosis, and suppurative bronchitis and bronchiolitis. This organism may opportunistically colonize lungs with chronically damaged respiratory defenses, such as occurs with enzootic calf pneumonia or existing lung lesions of feedlot cattle8.
Histophilus somni
Histophilus somni is being increasingly recognized as an important pathogen in BRD even though its prevalence is typically lower than M. haemolytica and/or P. multocida in both dairy and beef cattle13-15. These bacteria are normal inhabitants of the nasopharynx of cattle. However, infection of the lungs results in purulent bronchopneumonia that may be followed by septicemia and infection of multiple organs. H. somni is associated with extensive fibrinous pleuritis in feedlot calves. Pulmonary abscessation can occur as the pneumonia becomes chronic. H. somni may invade the lung and cause pneumonia after damage to the respiratory defenses. This organism can cause acute, often fatal septicemia. The systemic spread from the lung to the brain, myocardium, synovium, pleural and pericardial surfaces cause death between 40–60 days after arrival. The surviving animals can be subject to reproductive problems such as infertility8.

Mycoplasma bovis
Although 13 Mycoplasma species can be found in cattle, M. bovis is considered the most relevant species, especially in pneumonia in calves or adult cattle. M. bovis is a cell-wall-less bacterial pathogen, included in the class of Mollicutes, and is considered an emerging cause of respiratory disease and arthritis in feedlot cattle and in young dairy calves. Traditionally, M. bovis has most typically been linked to chronic BRD with characteristic pneumonic lesions that are often non-responsive to antimicrobial therapy. Recently, M. bovis receives much attention regarding its capability to be involved in acute cases of bronchopneumonia with recent evidence emerging linking M. bovis presence in the upper respiratory tract with acute BRD status16. After infection, M. bovis can spread through the bloodstream, establishing a long-term persistent infection by escaping the immune response. M. bovis, as primary agent or under the action of concomitant stressing factors such as weaning, transport, or relocation to feedlots, can impair the host immune system efficiency resulting in the onset of the disease including severe, often fatal, pneumonia. Case fatality is estimated to be 5–10% or higher in more severe cases, with morbidity reaching 35%. Lesions include chronic bronchopneumonia with caseous and coagulative necrosis. In severe cases, >80% of the lung tissue may be involved. The culture of these organisms requires special media and conditions; the growth of the organisms may take up to a week. PCR tests are now available that can detect mycoplasma within hours, thus greatly speeding up diagnosis. Several vaccines are available for M. bovis, mainly in North America, but their efficacy has not been demonstrated conclusively17. Thus, antimicrobial therapy remains the main control measure for the treatment of M. bovis pneumonia in cattle.
References
1. Maier GU, Love WJ, Karle BM, Dubrovsky SA, Williams DR, Champagne JD, et al. Management factors associated with bovine respiratory disease in preweaned calves on California dairies: The BRD 100 study. J Dairy Sci. 2019;102(8).
2. Overton MW. Economics of respiratory disease in dairy replacement heifers. Vol. 21, Animal Health Research Reviews. 2020.
3. Blakebrough-Hall C, McMeniman JP, González LA. An evaluation of the economic effects of bovine respiratory disease on animal performance, carcass traits, and economic outcomes in feedlot cattle defined using four BRD diagnosis methods. J Anim Sci. 2020;98(2).
4. Scott P. Respiratory Disease in Dairy and Beef Rearer Units. 2022.
5. Griffin D, Chengappa MM, Kuszak J, McVey DS. Bacterial pathogens of the bovine respiratory disease complex. Vol. 26, Veterinary Clinics of North America - Food Animal Practice. 2010.
6. Panciera RJ, Confer AW. Pathogenesis and pathology of bovine pneumonia. Vol. 26, Veterinary Clinics of North America - Food Animal Practice. 2010.
7. Timsit E, Hallewell J, Booker C, Tison N, Amat S, Alexander TW. Prevalence and antimicrobial susceptibility of Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni isolated from the lower respiratory tract of healthy feedlot cattle and those diagnosed with bovine respiratory disease. Vet Microbiol. 2017;208:118-125.
8. Gaudino M, Nagamine B, Ducatez MF, Meyer G. Understanding the mechanisms of viral and bacterial coinfections in bovine respiratory disease: a comprehensive literature review of experimental evidence. Vet Res. 2022 Sep 6;53(1):70.
9. Diep BA, Chan L, Tattevin P, Kajikawa O, Martin TR, Basuino L, et al. Polymorphonuclear leukocytes mediate Staphylococcus aureus Panton-Valentine leukocidin-induced lung inflammation and injury. Proc Natl Acad Sci U S A. 2010;107(12).
10. Lachowicz-Wolak A, Klimowicz-Bodys MD, Płoneczka-Janeczko K, Bykowy M, Siedlecka M, Cinciała J, et al. The Prevalence, Coexistence, and Correlations between Seven Pathogens Detected by a PCR Method from South-Western Poland Dairy Cattle Suffering from Bovine Respiratory Disease. Microorganisms. 2022 Jul 24;10(8):1487.
11. All-Island Animal Disease Surveillance Report 2018; http://www.animalhealthsurveillance.agriculture.gov.ie/
12. Becker CAM, Ambroset C, Huleux A, Vialatte A, Colin A, Tricot A, et al. Monitoring mycoplasma bovis diversity and antimicrobial susceptibility in calf feedlots undergoing a respiratory disease outbreak. Pathogens. 2020;9(7).
13. Angen Ø, Thomsen J, Larsen LE, Larsen J, Kokotovic B, Heegaard PMH, et al. Respiratory disease in calves: Microbiological investigations on trans-tracheally aspirated bronchoalveolar fluid and acute phase protein response. Vet Microbiol. 2009;137(1–2).
14. Fulton RW. Bovine respiratory disease research (1983-2009). Vol. 10, Animal health research reviews / Conference of Research Workers in Animal Diseases. 2009.
15. Murray GM, More SJ, Sammin D, Casey MJ, McElroy MC, O’Neill RG, et al. Pathogens, patterns of pneumonia, and epidemiologic risk factors associated with respiratory disease in recently weaned cattle in Ireland. Journal of Veterinary Diagnostic Investigation. 2017;29(1).
16. Valeris-Chacin R, Powledge S, McAtee T, Morley PS, Richeson J. Mycoplasma bovis is associated with Mannheimia haemolytica during acute bovine respiratory disease in feedlot cattle. Front Microbiol. 2022 Aug 1;13.
17. Dudek K, Szacawa E, Nicholas RAJ. Recent Developments in Vaccines for Bovine Mycoplasmoses Caused by Mycoplasma bovis and Mycoplasma mycoides subsp. mycoides. Vaccines (Basel). 2021;9(6):549.
About the author
Damien Achard (Ruminants Global Technical Manager)
Seasoned veterinarian, graduated from Ecole Nationale Vétérinaire de Nantes (France). After three years as a practitioner in central France, he pursued specialization in large animal internal medicine, completing an ACVIM residency and a Master of Sciences at the University of Montréal (2010-2014). Joining Semex Alliance as Health Manager for an IVF unit (2015-2016), he then transitioned to Ceva in 2016 as a Ruminants Global Technical Manager. Dr. Achard is an accomplished researcher, publishing on topics like downer cows, calf pneumonia or cryptosporidiosis and their associated therapies, and rational use of anthelmintics in ruminants. His ResearchGate profile (https://www.researchgate.net/profile/Damien-Achard/research) highlights his significant contributions to the veterinary field.
Explore author’s articlesAbout the author
Viviana Parreño (Biochemist - PhD Veterinary immunology and virology)
Dr Parreño is an expert in viral pathogens associated with neonatal diarrhoea in human and animal species (Rotavirus, Norovirus, Coronavirus), her team conduct epidemiological surveys, diagnosis, and molecular characterisation of enteropathogens. She also works in the development of passive immune strategies based on chicken egg yolk IgY and llama-derived nanobodies (recombinant monoclonal nano antibodies). She worked as well in the development and testing of viral vaccines in animal models (mice, calves, gnotobiotic pigs) and conducted the development and statistical validation of a guinea pig model as an alternative method for vaccine potency testing. The method was adopted as the official control in Argentina of combined vaccines applied in cattle containing IBR, BVDV, PI3, RVA and CoV.

-1.webp)


Leave your comments here