Bovine Viral Diarrhea (BVD) is a contagious viral disease of cattle. Two species of viruses belonging to the genus Pestivirus can be responsible for the disease. These are the BVDV-1 and BVDV-2 species, also known as Pestivirus A and Pestivirus B. In North America, the prevalence of BVDV-1 and BVDV-2 is about the same. In Europe, on the other hand, the BVD-2 virus is much rarer.
Several vaccines exist to prevent BVD. There are inactivated vaccines and modified live vaccines. In Europe, most of these vaccines are monovalent with a strain of BVDV-1. However, depending on the vaccine strain of BVDV-1 used and the vaccine technology, animals may also be protected against BVDV-2. This is called cross-protection. Several studies have shown this for a live vaccine containing the Oregon C24 strain of the BVDV-1 species.
Prefer to listen to this article? Click the play button below and enjoy our podcast!
Oregon C24 Modified Live Vaccine (BVDV-1) avoids clinical signs of BVDV-2 infection
An experimental study (Thibault et al., 2004) demonstrated the efficacy of this vaccine in preventing clinical signs following infection with a BVDV-2 virus. Fifteen calves were included in this study: five were vaccinated with the live vaccine, five were vaccinated with a bivalent inactivated vaccine (BVDV-1 and Border Disease virus) and five were not vaccinated and served as control. 61 days after the injection of the live vaccine or 29 days after the second injection of the inactivated vaccine, the calves were artificially infected intranasally with a strain of BVDV-2.
The animals were then followed clinically for up to 23 days. The clinical examination, assessed according to the Cortese scoring system, included observation of behaviour, tendency to haemorrhage, respiratory symptoms and diarrhoea. Points for these criteria were awarded as follows: no sign = 0, minor sign = 1, moderate sign = 2, severe sign = 3. The total score for each calf was calculated by adding up the individual points. Blood samples were also taken to assess leukopenia and to test for the presence of the BVDV-2 virus by RT-PCR in leukocytes.
Results
From a clinical point of view, none of the calves vaccinated with the live vaccine had hyperthermia above 39.5°C, unlike the control group. Regarding clinical score, for the animals in the live vaccine group, it remained zero except between the 8th and 11th day after infection when it peaked at 3. In comparison, the animals in the control group had a non-zero clinical score for 15 days, with a maximum of 12. Animals in the inactivated vaccine group had a non-zero clinical score for 11 days with a maximum of 5.

For haematological data, four of the five control animals manifested marked leukopenia (less than 4000 leukocytes/pl). In the group vaccinated with the inactivated vaccine, one calf developed leukopenia, while no animals had leukopenia in the group vaccinated with the live vaccine.
For RT-PCR detection, all animals in the control group were positive for at least 5 days and up to 12 days. In the group vaccinated with the inactivated vaccine, BVDV-2 virus was detected by RT-PCR in two animals while no animals were viremic in the group vaccinated with the live vaccine (Table 1).
| Days after infection | Control group | Inactivated vaccine group | Modified live vaccine group |
| 0 | - | - | - |
| 2 | - | - | - |
| 5 | 5 | 1 | - |
| 7 | 5 | 3 | - |
| 9 | 5 | 1 | - |
| 12 | 3 | - | - |
| 14 | 1 | - | - |
| 16 | 1 | - | - |
| 19 | - | - | - |
| 23 | - | - | - |
Table 1: Number of viremic animals per day and per group following experimental challenge
Oregon C24 Modified Live Vaccine produces an equivocal neutralising antibody level against BVD-1 and BVDV-2
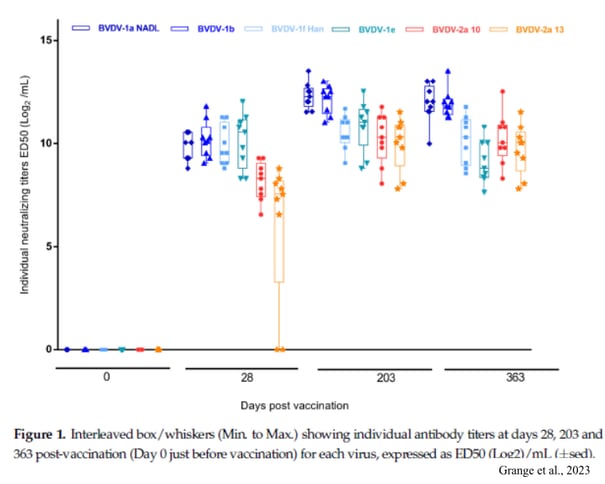
A more recent study (Grange et al., 2023) assessed the neutralisation of two strains of BVDV-2a virus by serum from heifers vaccinated with the live vaccine. A comparison was also made with four strains of BVDV-1 (1a NADL, 1b, 1e and 1f Hanover). Sera were collected from 9 heifers on days 28, 203 and 363 after vaccination. Sero-neutralisation tests were carried out for the 6 BVDV strains on each collection day and for all the animals.
All strains were neutralised by the sera from D28 after vaccination except for two heifers whose serum did not neutralise one of the two strains of BVDV-2a at this date. However, at D203, their serum correctly neutralised all strains (figure 1). It is particularly interesting to note that the titer of neutralising antibodies against the BVDV-2 strains is similar to the titer against the BVDV-1f Hanover strain used to challenge pregnant females 12 months after vaccination (Achard et al., 2018). In this study, no vaccinated female had aborted or given birth to an infected calf, showing that the vaccine is effective in protecting the foetus in the event of infection of its mother during pregnancy. Therefore, it can be hypothesise that vaccination with a live vaccine containing the BVDV-1 Oregon C24 strain provides humoral cross-over immunity, which may provide foetal protection against both BVDV-1 and BVDV-2a infections.
Key messages
- Modified live BVD vaccines are more effective than inactivated vaccines in protecting animals against heterologous strains.
- Serum from animals vaccinated with a modified live vaccine containing the Oregon C24 BVD-1 strain is capable of neutralising multiple strains of BVDV, including BVDV-2a.
- Neutralisation titres against BVDV-2a strains are similar to those against BVDV-1 strains after vaccination.
References
Achard, D., Munoz-Bielsa, J., Pinho, P., & Febery, E. (2018, August). Determination of the fetal protection in pregnant heifers challenged with bovine viral diarrhea type 1 virus twelve months after one administration of a live-attenuated vaccine. In Proceedings of the 30th World Buiatrics Congress, Sapporo, Japan (pp. 27-31).
Grange, G., Mindeguia, M., Gisbert, P., & Meyer, G. (2023). Cross-neutralization between bovine viral diarrhea virus (BVDV) types 1 and 2 after vaccination with a BVDV-1a modified-live-vaccine. Vaccines, 11(7), 1204.
Thibault, J. C., Hamers, C., Couvreur, B., Letellier, C., Dehan, P., Brun, A., ... & Kerkhofs, P. (2004). Untersuchung zur Wirksamkeit der Impfung mit einem BVDV1-Lebendimpfstoff sowie mit einem inaktivierten BVDV1-Impfstoff gegen eine BVDV2-Testinfektion. Tierarztliche Umschau, 59(3).
About the author
Philippe Gisbert (Ruminants Global Technical Manager)
Philippe Gisbert started his career in 1994 as a Vet practitioner working with companion and farm animals for over 9 years. He then became Health Affairs Manager for Group Agena (artificial insemination company). In 2008 he joined Eurofins – Laboratoire Coeur de France as Animal Health Unit Manager where he worked for 7 years until he joined Ceva France as Technical Manager Ruminants (Infectiology, Vaccines and Diagnostic). Since 2020 he is Global Technical Manager for Biologicals, Udder Health and Antiinflammatories. He is a member of SIMV diagnostic and anti-infective technical groups and has integrated different working groups of ANSES and UNCEIA related to epidemiology, antibiotic resistance and reproduction in livestock.
Explore author’s articles



Leave your comments here